Printable Clia Form Cms 116 Back to CMS Forms List CMS 116 Dynamic List Information Dynamic List Data Form CMS 116 Form Title CLINICAL LABORATORY IMPROVEMENT AMENDMENTS OF 1988 CLIA APPLICATION FOR CERTIFICATION Revision Date 2020 04 01 O M B 0938 0581 O M B Expiration Date 2024 03 31 Special Instructions N A
First download the CMS 116 Clinical Laboratory Improvement Amendments CLIA application form https www cms gov Medicare CMS Forms CMS Forms Downloads CMS116 pdf Section I In section I of this CMS 116 form complete the basic information regarding your laboratory and director Section II Download Fillable Form Cms 116 In Pdf The Latest Version Applicable For 2024 Fill Out The Clinical Laboratory Improvement Amendments clia Application For Certification Online And Print It Out For Free Form Cms 116 Is Often Used In U s Department Of Health And Human Services Centers For Medicare And Medicaid Services U s Department Of Health And Human Services United States Federal
Printable Clia Form Cms 116

Printable Clia Form Cms 116
https://data.templateroller.com/pdf_docs_html/1735/17356/1735685/page_3_thumb_950.png

CMS 116 2007 Fill And Sign Printable Template Online US Legal Forms
https://www.pdffiller.com/preview/14/848/14848242/large.png

How To Apply For A CLIA Certificate Filling Out CMS 116 Lighthouse Lab Services
https://www.lighthouselabservices.com/wp-content/uploads/2020/03/1-4.jpg
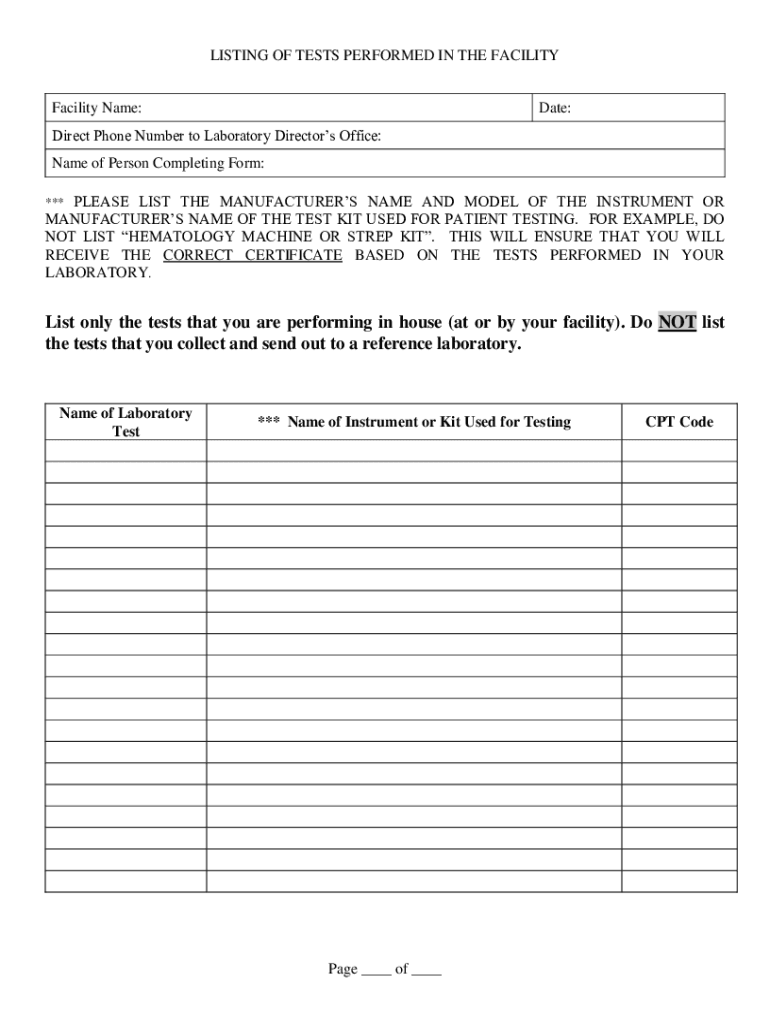
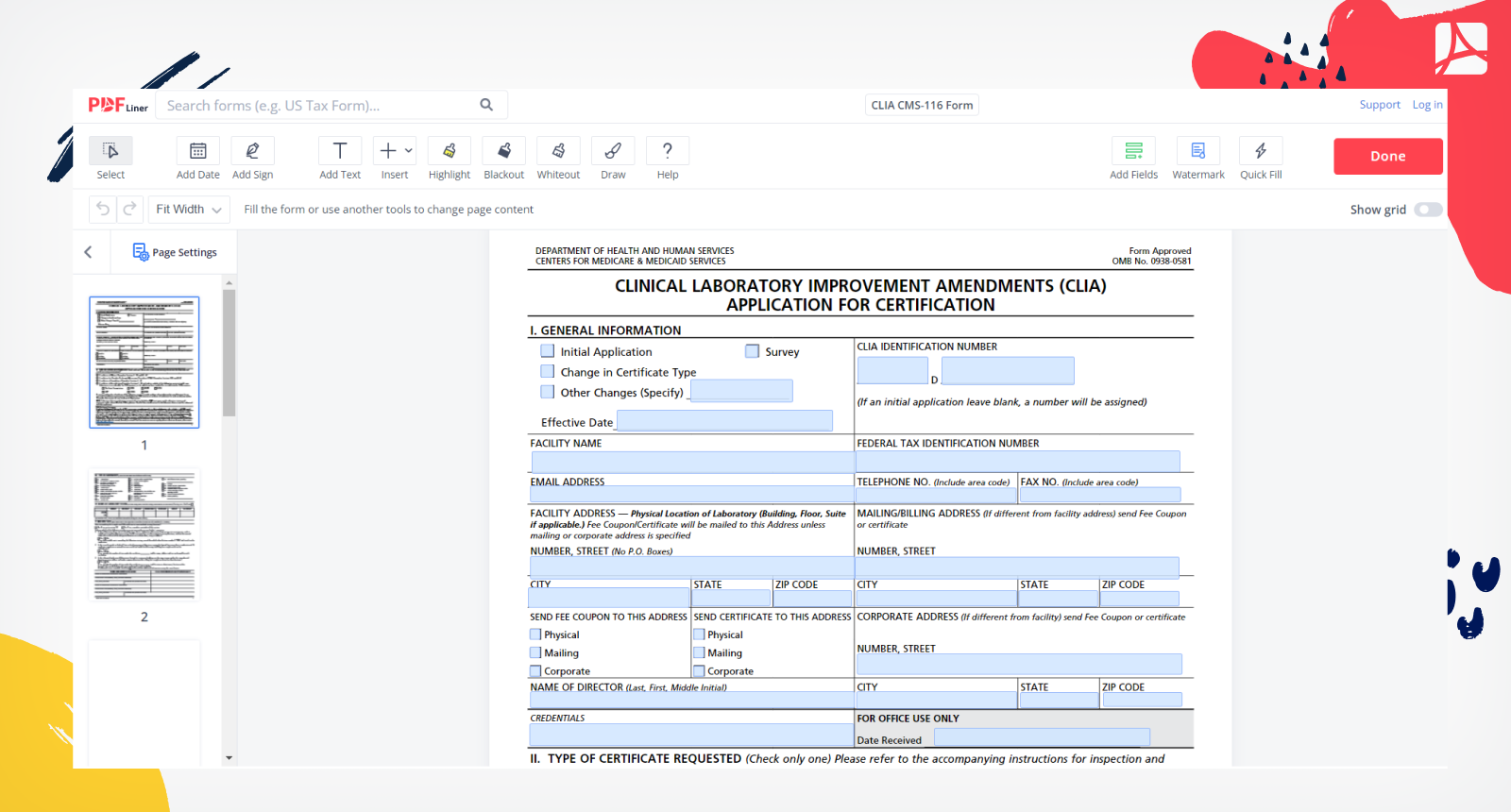
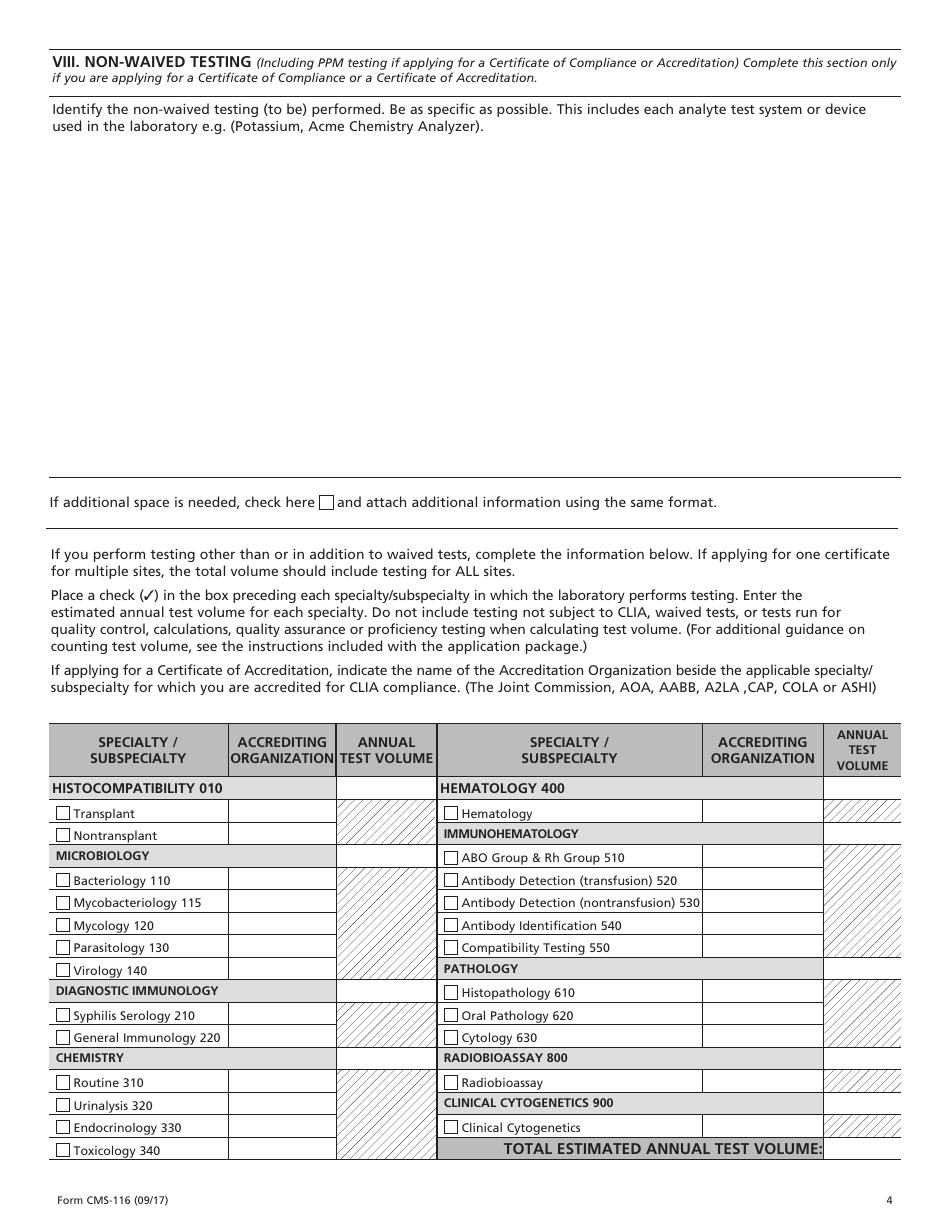
The CLIA application Form CMS 116 collects information about your laboratory s operation which is necessary to determine the fees to be assessed to establish baseline data and to fulfill the statutory requirements for CLIA This information will also provide an overview of your facility s laboratory operation Form CMS 116 07 05 Previous Versions Obsolete EF 07 2005 Page 1 of 4 III TYPE FORM CMS 116 INSTRUCTIONS FOR COMPLETION CLIA requires every facility that tests human specimens for the purpose of providing information for the diagnosis PRINT LEGIBLY OR TYPE INFORMATION
1 Complete Form CMS 116 Clinical Laboratory Improvement Amendments CLIA Application for Certification and mail it to the appropriate CLIA State Agency 2 Pay applicable fees based on certification type For moderate and high complexity laboratories additional fees are based on annual testing volume and scope of testing 3 Submit the CMS 116 form PDF and other required documents to your State Agency PDF which will process your application What Credentials Do I Need to Apply Directors Who Perform Non Waived Testing
More picture related to Printable Clia Form Cms 116
Clia Application Texas Form Fill Out And Sign Printable PDF Template SignNow
https://www.signnow.com/preview/201/622/201622905/large.png
Clia Application Cms 116 Form Fill Out Printable PDF Forms Online
https://formspal.com/data/LandingPageImages/Image/2/206/206793.JPEG

Cms 100 Fill Online Printable Fillable Blank PdfFiller
https://www.pdffiller.com/preview/100/551/100551352/large.png
Download and Complete Form CMS 116 The CLIA application Form CMS 116 collects information about your facility s e g school operation to issue a CLIA number Include information based on the date of form completion All applicable highlighted sections fields must be completed Incomplete applications cannot be processed Print legibly or type LabExcellence cms hhs gov Form CMS 116 09 17 Form CMS 116 09 17 2 CITY STATE ZIP CODE PRINT NAME OF OWNER DIRECTOR OF LABORATORY Completed forms can be submitted via email to Dph Clia Illinois gov faxed to or laboratory certification AND AND AND clinical
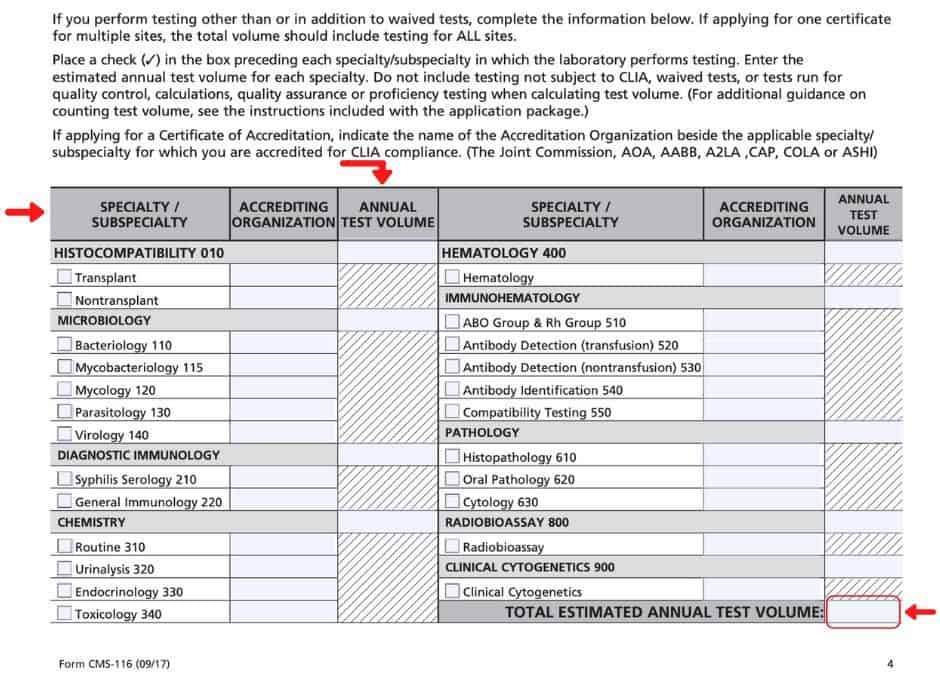
Published February 13 2023 Courtney Bishnoi Earlier this month the CMS CLIA office released memorandum 23 05 CLIA outlining procedural guidance for the use of form CMS 116 The memo summarizes what laboratory changes require a new CMS 116 form to be completed and when written notification of a change is sufficient The CLIA application Form CMS 116 collects XYZ Hospital For a physician s office this may be the information about your laboratory s operation which name of the physician NOTE the information provided is necessary to determine the fees to be assessed to is what will appear on your certificate

Fillable Form Cms 116 Clinical Laboratory Improvement Amendments Of 1988 Clia Application
https://data.formsbank.com/pdf_docs_html/302/3026/302688/page_1_thumb_big.png

How To Apply For A CLIA Certificate Filling Out CMS 116 Form YouTube
https://i.ytimg.com/vi/_OMI5ujn_iI/maxresdefault.jpg

https://www.cms.gov/Medicare/CMS-Forms/CMS-Forms/CMS-Forms-Items/CMS012169
Back to CMS Forms List CMS 116 Dynamic List Information Dynamic List Data Form CMS 116 Form Title CLINICAL LABORATORY IMPROVEMENT AMENDMENTS OF 1988 CLIA APPLICATION FOR CERTIFICATION Revision Date 2020 04 01 O M B 0938 0581 O M B Expiration Date 2024 03 31 Special Instructions N A

https://www.lighthouselabservices.com/clia-certificate-filling-out-cms-116/
First download the CMS 116 Clinical Laboratory Improvement Amendments CLIA application form https www cms gov Medicare CMS Forms CMS Forms Downloads CMS116 pdf Section I In section I of this CMS 116 form complete the basic information regarding your laboratory and director Section II
How To Apply For A CLIA Certificate Filling Out CMS 116 Lighthouse Lab Services

Fillable Form Cms 116 Clinical Laboratory Improvement Amendments Of 1988 Clia Application
Clia Application Cms 116 Form Fill Out Printable PDF Forms Online
Clia Application Cms 116 Form Fill Out Printable PDF Forms Online

2017 Form CMS 116 Fill Online Printable Fillable Blank PdfFiller
CMS 116 CLIA Application Form Blank Sign Forms Online PDFliner

CMS 116 CLIA Application Form Blank Sign Forms Online PDFliner

Fillable Online CMS Releases Procedural Guidance For CLIA Form CMS 116 Fax Email Print PdfFiller

2020 2023 Form CMS 116 Fill Online Printable Fillable Blank PdfFiller
Form CMS 116 Download Fillable PDF Or Fill Online Clinical Laboratory Improvement Amendments
Printable Clia Form Cms 116 - How to obtain a CLIA Certificate NOTE Congress passed the Clinical Laboratory Improvement Amendments CLIA in 1988 establishing authority to promulgate standards for certain laboratory testing to ensure the accuracy reliability and timeliness of test results regardless of where or by whom the test was performed
